Introduction of Magnesium fluoride MgF2
Magnesium fluoride is an inorganic compound with the formula MgF2. The compound is a white crystalline salt and is transparent over a wide range of wavelengths, with commercial uses in optics that are also used in space telescopes.
Magnesium (atomic symbol: Mg, atomic number: 12) is a Block S, Group 2, Period 3 element with an atomic mass of 24.3050. The number of electrons in each of Magnesium's shells is [2, 8, 2] and its electron configuration is [Ne] 3s2. The magnesium atom has a radius of 160 pm and a Van der Waals radius of 173 pm. Magnesium was discovered by Joseph Black in 1775 and first isolated by Sir Humphrey Davy in 1808. Magnesium is the eighth most abundant element in the earth's crust and the fourth most common element in the earth as a whole. Elemental MagnesiumIn its elemental form, magnesium has a shiny grey metallic appearance and is an extremely reactive. It is can be found in minerals such as brucite, carnallite, dolomite, magnesite, olivine and talc. Commercially, magnesium is primarily used in the creation of strong and lightweight aluminum-magnesium alloys, which have numerous advantages in industrial applications. The name "Magnesium" originates from a Greek district in Thessaly called Magnesia.
Fluorine is a Block P, Group 17, Period 2 element. Its electron configuration is [He]2s22p5. The fluorine atom has a covalent radius of 64 pm and its Van der Waals radius is 135 pm. In its elemental form, CAS 7782-41-4, fluorine gas has a pale yellow appearance. Fluorine was discovered by André-Marie Ampère in 1810. It was first isolated by Henri Moissan in 1886.
Magnesium fluoride is highly insoluble in water (0.0076 g/100 mL) at 18°C [151]. It can be agglomerated with water, pressed into green pellets, dewatered, and sintered at high temperatures to produce porous pellets that can be used to selectively trap technetium. Trapped technetium cannot be easily desorbed from the MgF2, requiring temperatures above 1000°C. However, trapped technetium can be easily removed by washing with water or dilute nitric acid. The magnesium fluoride can be reused after a drying step [152]. Magnesium fluoride has been used at a large scale for the selective trapping of volatile technetium fluorides and oxyfluorides of technetium comingled with UF6 [22,152,153].
Magnesium fluoride (MgF2)-containing coatings were prepared on biodegradable Mg-based metals through hydrofluoric acid-involving chemical conversion (Chiu, Wong, Cheng, & Man, 2007; Lin, Tan, Wan et al., 2013; Pereda et al., 2010; Thomann et al., 2010; Yan et al., 2010) or MAO (Pan, Chen, Wang, & Lin, 2013; Pan, Chen, Wang, & Zhao, 2013; Seyfoori, Mirdamadi, Khavandi, & Raufi, 2012) methods. Fluorine (F) is a natural component in human bones and teeth (Zheng, Wu, Ng, Wang, & Lian, 2002). A proper release of F will not cause harm to the organisms (Thomann et al., 2010). The bone response to fluoride is dose-dependent: a low-dose release of F will facilitate the bone formation; however, a high dose of it will cause the formation of poorly mineralized osteoid (Ellingsen, 1995). Thus, MgF2 is an acceptable coating material, although the degradation rate should be controlled.
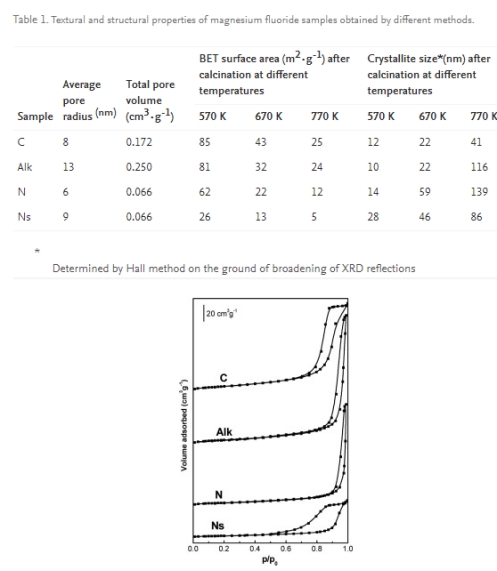
Magnesium fluoride was prepared using four different methods, however, thermal treatment of all samples was identical and consisted in calcination at 670 K. Despite the same calcination temperature, MgF2 samples considerably differ in their porous structure which is reflected in the shape of hysteresis loop in the isotherm of low-temperature nitrogen adsorption, specific surface area, pore size and pore volume (Table 1; Figure 1). The greatest surface area (43 m2·g-1) was obtained in the case of carbonate sample (C), whereas the smallest one (13 m2·g-1) in the case of spherical MgF2 prepared from magnesium nitrate (Ns). Adsorption-desorption isotherms of all samples are of type IV, however, they differ in the shape of their hysteresis loops. The latter belong to type H1 for samples C, Alk and N, which indicates the presence of cylindrical pores, whereas hysteresis loop of the sample Ns combines features of hysteresis loops of types H1 and H2, encountered when narrow-necked pores are present. The greatest pore size (r = 13 nm) and pore volume (0.250 cm3·g-1) were found in magnesium fluoride prepared from magnesium alkoxide (Alk). The discussed sample is also characterized by the lowest thermal stability as concluded from considerable increase in crystallite size and drastic reduction in surface area with the rise in calcination temperature (Table 1). The highest resistance to sintering and recrystallisation at high temperatures were shown by carbonate (C) and nitrate-spherical (Ns)samples.
Part of the content of this article comes from Science Direct :https://www.sciencedirect.com
FOSHAN NANHAI SHUANGFU CHEMICAL CO., LTD is one of the manufacture of medium and high quality Magnesium fluoride, supply long term production and delivery of this product all over the world.
Attn:Nicole Huang (Sales Manager)
Add: Qishan Industry Park, Yanfeng Road, Shishan, Nanhai District,Foshan City,China.
Phone: 86 757 81108788
Mobile: 86 13928083637
E-mail: sales@df-chemicals.com




