Why Potassium Hexafluorotitanate (K2TiF6) is Used in the Preparation of High-Efficiency Optoelectronic Materials
Potassium hexafluorotitanate (K2TiF6) is a chemical compound with a crucial role in the development of high-efficiency optoelectronic materials. It is particularly valued in the fabrication of solar cells, LEDs, and other devices that rely on the efficient management of light and electrical charge. This article delves into the reasons K2TiF6 is chosen for such applications, explaining the chemical reactions it undergoes and how these reactions translate to improved material performance.
1、K2iF6 as a Titanium Source for Optoelectronic Applications
One of the primary reasons for the use of K2TiF6 in optoelectronic material preparation is its ability to provide titanium in a controlled and high-purity form. Titanium is essential in materials like titanium dioxide (TiO2), which are commonly used in dye-sensitized solar cells (DSSCs), perovskite solar cells (PSCs), and photodetectors. TiO2 serves as a charge transport layer due to its excellent electron mobility and wide bandgap.
The key chemical reaction through which K2TiF4 contributes to this process involves hydrolysis. In the presence of water, K2TiF6 dissociates to release titanium ions (Ti4+):
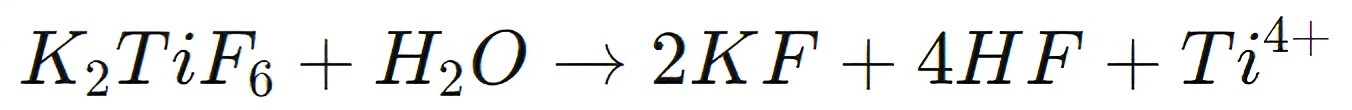
This reaction creates a precursor for the formation of TiO2, which is critical for optoelectronic devices. After hydrolysis, the resulting titanium hydroxide (Ti(OH)4) undergoes further thermal treatment to form TiO2:
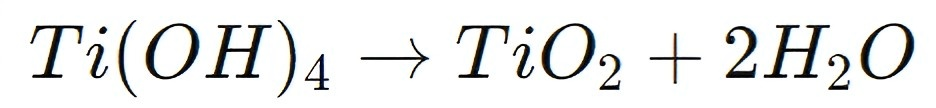
This process yields high-purity titanium dioxide, which is essential for ensuring efficient charge transport and minimal recombination losses in optoelectronic devices. In solar cells, TiO2 derived from K2TiF6 serves as an electron transport layer (ETL), facilitating the smooth flow of electrons from the light-absorbing layer to the electrodes.
2、Improvement in Crystal Structure and Defect Passivation
The efficiency of optoelectronic devices such as solar cells and LEDs is heavily dependent on the quality of the material’s crystal structure. A well-ordered crystal lattice allows for efficient light absorption and charge transport, while defects in the crystal can trap charge carriers, leading to energy losses. K2TiF6 plays a critical role in enhancing the crystallinity of materials, particularly perovskites, which are used in high-performance solar cells.
In perovskite solar cells (PSCs), K2TiF6 is used to control the crystal growth process during the formation of the light-absorbing perovskite layer. The presence of fluorine ions (F-) from K2TiF6 contributes to defect passivation by binding to unsaturated bonds at the crystal surface. This process is essential for improving the material’s optoelectronic properties, as it reduces the number of defect sites where electron-hole recombination can occur.
The chemical reaction responsible for this defect passivation is as follows:
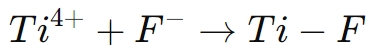
The formation of Ti-F bonds on the perovskite surface ensures that the material's electronic properties are optimized, reducing energy losses and enhancing the efficiency of solar cells. Additionally, the presence of fluorine atoms improves the film’s uniformity, which is crucial for achieving high-performance optoelectronic devices.
3、Surface and Interface Passivation for Enhanced Stability
One of the significant challenges in optoelectronic materials is their stability. Many materials, particularly perovskites, degrade when exposed to moisture, heat, or UV light, limiting their lifespan and efficiency. K2TiF6 helps improve the stability of these materials through a process known as surface and interface passivation.
The chemical principle behind this process is the strong bond formed between titanium and fluorine (Ti-F bonds), which is highly resistant to environmental degradation. By incorporating K2TiF6 during the material preparation process, a stable Ti-F layer forms on the surface of the optoelectronic material, providing a protective barrier against moisture and oxygen. This passivation layer prevents the material from undergoing hydrolysis or oxidation, both of which can significantly impair device performance.
The key chemical reaction involved in this passivation process is:
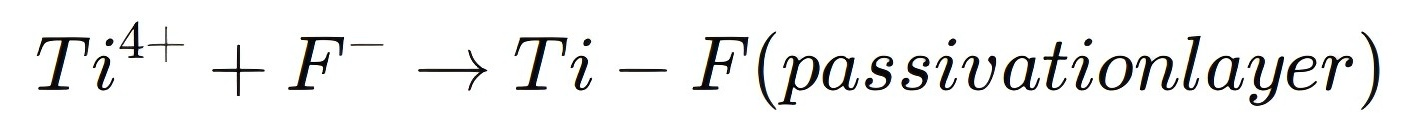
This chemical reaction creates a highly stable titanium-fluorine complex that shields the material from environmental factors, improving both the stability and longevity of optoelectronic devices.
4、Optimizing Charge Carrier Dynamics
In high-efficiency optoelectronic devices, such as solar cells and LEDs, the movement of charge carriers (electrons and holes) plays a critical role in determining device performance. K2TiF6 is often used to optimize the dynamics of these charge carriers by improving the conductivity of materials and minimizing charge recombination.
One way K2TiF6 achieves this is by reducing surface defects that can trap charge carriers. The fluorine ions from K2TiF6 interact with unsaturated bonds at the material’s surface, creating a smoother, more defect-free interface. This process ensures that the electrons and holes generated within the material are efficiently transported to the electrodes without being lost to recombination.
The following reaction illustrates the passivation of surface defects:
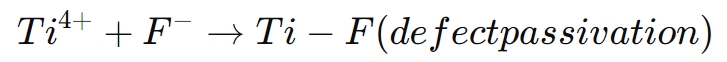
This interaction reduces the likelihood of electron-hole recombination, leading to higher charge carrier mobility and improved conductivity. In solar cells, this results in a higher short-circuit current and open-circuit voltage, both of which contribute to a higher overall power conversion efficiency.
5、Chemical Stability and Reaction Mechanisms in LEDs
K2TiF6 is also employed in the preparation of light-emitting diodes (LEDs), where it enhances the chemical stability and performance of the emissive layers. In LEDs, one of the primary challenges is preventing non-radiative recombination, a process in which charge carriers lose energy without emitting light. This reduces the efficiency of the LED and limits its brightness.
K2TiF6 helps address this issue by passivating defects in the emissive layer, particularly at grain boundaries where non-radiative recombination tends to occur. The fluorine ions from K2TiF6 form stable bonds with the material’s surface, preventing defect formation and improving the recombination efficiency of electrons and holes.
The key reaction that enhances LED performance is:
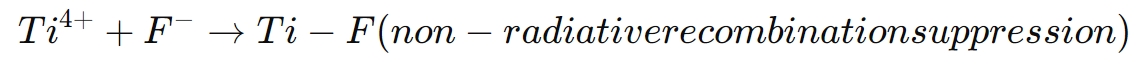
By passivating these defects, K2TiF6 ensures that more charge carriers recombine radiatively, emitting light and improving the LED’s overall efficiency. This results in brighter, more stable LEDs with longer operational lifetimes.

6、Interface Engineering in Solar Cells and Photodetectors
In addition to its role in the bulk material, K2TiF6 is also used in interface engineering to optimize the junctions between different layers in optoelectronic devices. The quality of these interfaces is crucial for device performance, as poor interfaces can lead to energy losses through charge recombination or reflection.
In solar cells, for example, K2TiF6 can be introduced at the interface between the perovskite active layer and the electron transport layer (ETL) to improve charge extraction. The fluorine ions from K2TiF6 help align the energy levels of the two layers, reducing the energy barrier for electron transfer. This results in more efficient charge extraction and a reduction in interfacial recombination.
The relevant chemical interaction is as follows:
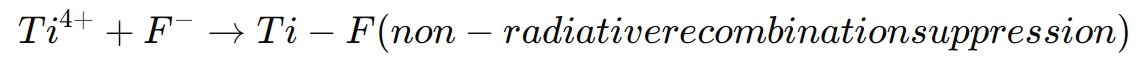
This reaction ensures that the energy levels of the different layers are properly aligned, facilitating smooth electron transport and improving the overall efficiency of the solar cell.
Potassium hexafluorotitanate (K2TiF6) is an invaluable chemical compound in the preparation of high-efficiency optoelectronic materials. Its ability to provide high-purity titanium sources, passivate surface and interface defects, and improve material stability makes it essential in the fabrication of devices such as solar cells, LEDs, and photodetectors. The chemical reactions facilitated by K2TiF6—such as the formation of Ti-F bonds and the passivation of defects—directly translate to enhanced performance, ensuring that optoelectronic materials operate efficiently and reliably over extended periods. Through these mechanisms, K2TiF6 continues to play a critical role in advancing the field of optoelectronics.




