Fluorosilicic Acid Unleashed: Transformative Roles and Principles in Revolutionizing Lead Refining
In the realm of lead refining, technological advancements continually reshape traditional practices. One such transformative agent is fluorosilicic acid, a compound with multifaceted applications in the lead industry. This article explores the innovative uses and underlying principles of fluorosilicic acid, shedding light on its pivotal role in modern lead refining processes.

Understanding Fluorosilicic Acid
Fluorosilicic acid, often derived from the production of phosphoric acid, has emerged as a versatile solution in various industrial applications. With its unique properties and chemical composition, fluorosilicic acid has found a niche in the lead refining sector, where it contributes to efficiency, environmental sustainability, and overall process improvement.
Applications in Lead Refining
1、Desulfurization
One primary application of fluorosilicic acid in lead refining lies in desulfurization. Lead ores often contain sulfur impurities that can compromise the quality of the final product. Fluorosilicic acid reacts with sulfur, forming stable compounds that can be easily separated from the lead, resulting in a purer end product.
2、Fluoride Removal
Fluoride is another common impurity in lead ores. Fluorosilicic acid acts as an effective agent for removing fluoride from the lead concentrate, ensuring compliance with stringent quality standards. This application is crucial in producing lead suitable for diverse industrial applications.
3、Lead Electrorefining
In electrorefining processes, fluorosilicic acid plays a vital role in optimizing electrolyte composition. By carefully controlling the concentration of fluorosilicic acid in the electrolyte, operators can enhance the efficiency of lead electrorefining, leading to improved energy consumption and reduced environmental impact.
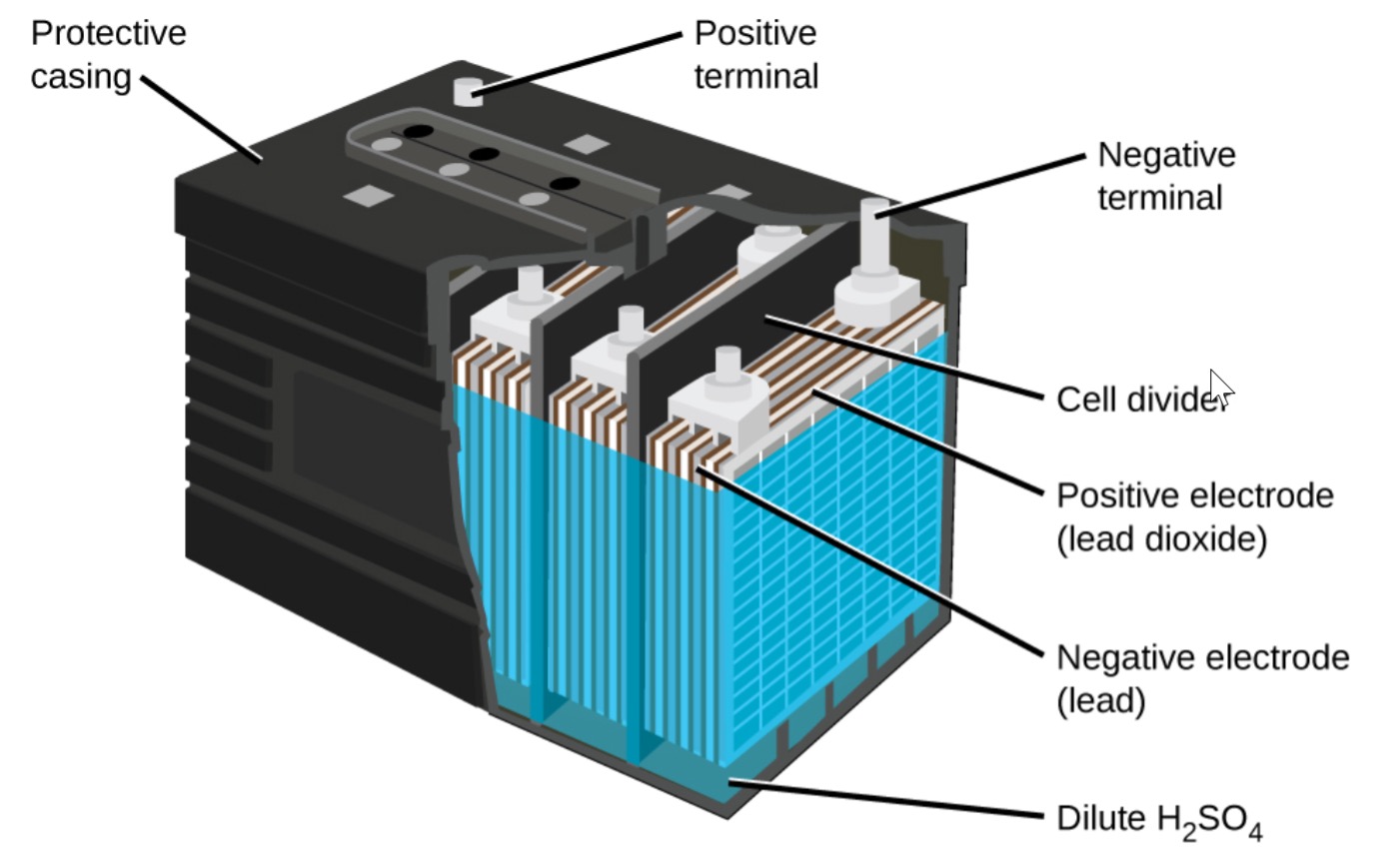
4、Lead-Acid Battery Manufacturing
Fluorosilicic acid is integral in the production of lead-acid batteries. It contributes to the formation of lead fluoride coatings on the electrodes, improving battery performance and longevity. This application underscores the far-reaching impact of fluosilicic acid in diverse sectors beyond primary lead refining.
Principles Behind the Applications
1、Chemical Reactions
The key to fluorosilicic acid's efficacy lies in its ability to engage in chemical reactions with impurities present in lead ores. The acid facilitates reactions that result in the formation of stable compounds, which can be easily separated from the lead matrix.
2、Selective Precipitation
Fluorosilicic acid enables selective precipitation of undesired elements, such as sulfur and fluoride, through carefully controlled pH adjustments. This selective process ensures minimal impact on other components in the ore, preserving the integrity of the lead concentrate.
3、pH Control in Electrolysis
In electrorefining, maintaining optimal pH levels is crucial for controlling the deposition of lead on the cathode. Fluorosilicic acid contributes to pH regulation, allowing for precise control over the electrorefining process and maximizing energy efficiency.
Fluorosilicic acid's integration into the lead refining industry marks a significant step towards sustainable and efficient practices. Its applications in desulfurization, fluoride removal, electrorefining, and battery manufacturing underscore its versatility. As the lead industry continues to evolve, fluosilicic acid stands as a beacon of innovation, driving advancements that benefit both the industry and the environment.
- Fluoride Salt
- Ammonium Fluoride
- Sodium Fluoride
- Potassium Fluoride
- Sodium Hydrogenfluoride
- Potassium Bifluoride
- Magnesium Fluoride
- Aluminium Fluoride
- Barium Fluoride
- Lithium Fluoride
- Strontium Fluoride
- Nickel Fluoride
- Zinc Fluoride




