Potassium Hydrogen Fluoride as an Intermediate in Chemical Production
Potassium Hydrogen Fluoride (KHF2), or potassium bifluoride, is a unique and versatile compound extensively used in chemical manufacturing. As an intermediate, KHF2 facilitates the production of a wide range of chemicals and materials. This article will explore the various ways in which KHF2 is employed as an intermediate in chemical processes, shedding light on its critical role in the synthesis of complex compounds and its contributions to modern industry.

Chemical Characteristics of Potassium Hydrogen Fluoride
Understanding the chemical characteristics of Potassium Hydrogen Fluoride is essential to grasp its functionality as an intermediate. KHF2 is an ionic compound composed of potassium ions (K+) and bifluoride ions (HF2-). The bifluoride ion is particularly interesting due to its ability to donate fluoride ions (F-) in chemical reactions, a feature that makes KHF2 an effective fluorinating agent.
KHF2 is produced by reacting potassium carbonate (K2CO3) or potassium hydroxide (KOH) with hydrofluoric acid (HF). The reaction is highly exothermic and yields KHF2 as a solid product:
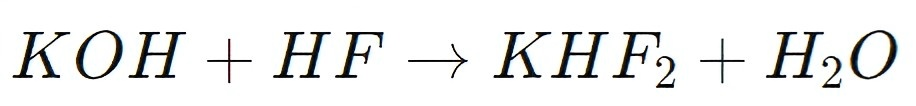
KHF2 as a Fluorinating Agent
1、Introduction of Fluorine into Organic Compounds
One of the most significant applications of Potassium Hydrogen Fluoride as an intermediate is its role in introducing fluorine atoms into organic molecules. Fluorine, with its unique properties such as high electronegativity and strong bond formation, significantly alters the chemical behavior of organic compounds. The introduction of fluorine can enhance the stability, bioavailability, and reactivity of organic molecules, making them valuable in pharmaceuticals, agrochemicals, and materials science.
KHF2 serves as a fluorinating agent, particularly in nucleophilic fluorination reactions. These reactions involve the replacement of other functional groups, such as hydroxyl (OH) or halogen (Cl, Br), with a fluoride ion from KHF2. This transformation is critical in the synthesis of various fluoroaromatics and aliphatic fluorides, which are precursors for more complex molecules.
For example, in the production of fluorinated pharmaceuticals, KHF2 is used to fluorinate aromatic compounds, which are then further processed to develop active pharmaceutical ingredients (APIs). This method is favored for its efficiency and the selectivity with which fluorine atoms are introduced into the target molecules.
2、Production of Fluorosilicic Acid
Another important application of Potassium Hydrogen Fluoride is in the production of fluorosilicic acid (H2SiF6), a compound widely used in water fluoridation, metal surface treatment, and as a raw material for producing aluminum fluoride (AlF3). The synthesis of fluorosilicic acid involves reacting silicon dioxide (SiO2) with KHF₂, where KHF2 provides the necessary fluoride ions for the reaction:
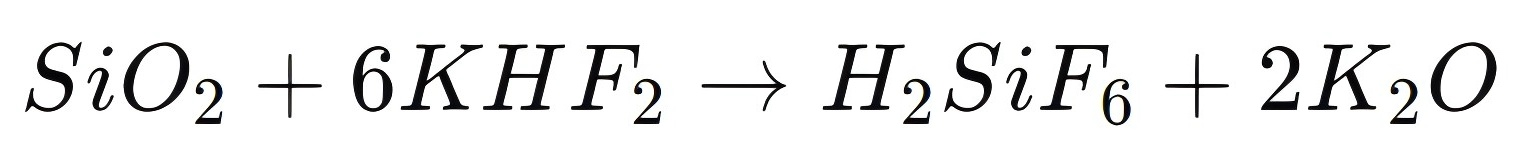
This process highlights the role of KHF₂ as a source of fluoride ions in industrial chemical reactions. Fluorosilicic acid, in turn, is an essential compound in the production of other fluorides and is used to add fluoride to drinking water, enhancing dental health in communities.
KHF2 in Inorganic Synthesis
1、Synthesis of Metal Fluorides
Potassium Hydrogen Fluoride is widely used in the synthesis of various metal fluorides, including those of aluminum, magnesium, and zirconium. These metal fluorides have diverse applications in industries such as ceramics, optics, and metallurgy. In the production of aluminum fluoride (AlF3), which is used as a flux in aluminum smelting, KHF2 is reacted with aluminum oxide (Al2O3):
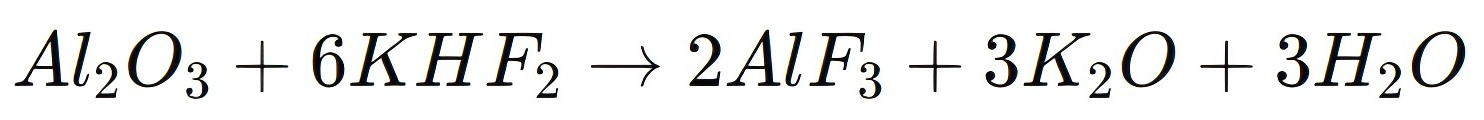
2、Preparation of Potassium Fluoroborate
KHF2 is also used in the preparation of potassium fluoroborate (KBF4), a compound employed in various metallurgical and chemical processes. Potassium fluoroborate is produced by reacting KHF2 with boric acid (H3BO3):
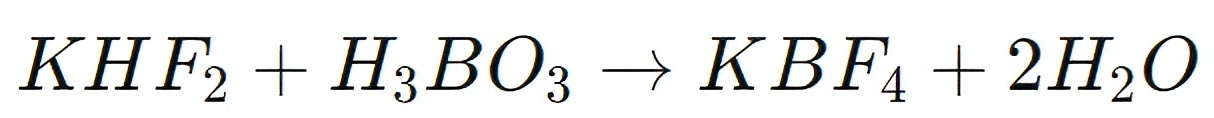
The resulting potassium fluoroborate is used as a flux in the aluminum industry, where it helps to lower the melting point of aluminum alloys and facilitates the removal of impurities during the casting process. Additionally, KBF4 is used in the production of boron-containing chemicals, which have applications in glass manufacturing, electronics, and as catalysts in organic synthesis.
KHF₂ in Hydrofluoric Acid Production
Hydrofluoric acid (HF) is a highly corrosive and reactive acid used in a variety of industrial processes, including glass etching, metal cleaning, and petrochemical refining. Potassium Hydrogen Fluoride is often used as an intermediate in the production of hydrofluoric acid, particularly in situations where a controlled release of HF is necessary.
KHF2 can be thermally decomposed to release hydrofluoric acid:
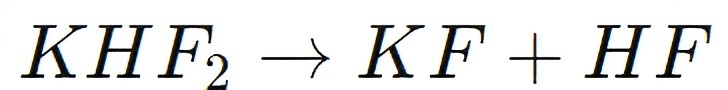
This decomposition process is employed in scenarios where the direct handling of HF gas is dangerous or impractical. By using KHF2 as a precursor, industries can produce HF on-site and on-demand, minimizing the risks associated with the storage and transport of hydrofluoric acid.
Application in Electrolytic Processes
Potassium Hydrogen Fluoride is also crucial in various electrolytic processes, particularly in the production of fluorine gas and other fluorine-containing compounds. In these processes, KHF2 acts as a conductive medium and a source of fluoride ions, which are essential for the electrolytic reactions.
For instance, in the production of fluorine gas (F2), KHF2 is dissolved in anhydrous hydrogen fluoride (AHF) and subjected to electrolysis. The process involves the passage of an electric current through the electrolyte, leading to the formation of fluorine gas at the anode and potassium fluoride at the cathode:
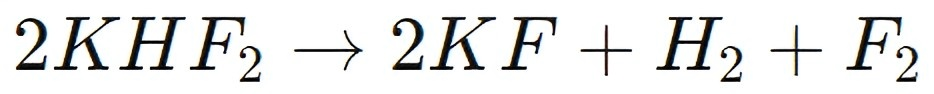
This method of producing fluorine gas is highly efficient and allows for the controlled generation of fluorine, which is used in the manufacture of high-performance materials such as Teflon (PTFE), refrigerants, and in uranium enrichment processes.
KHF2 in Catalysis
Potassium Hydrogen Fluoride is also utilized as a catalyst in various chemical reactions, particularly those involving the activation of fluorinated reagents. The presence of KHF2 can enhance the reactivity of certain compounds, making it easier to achieve the desired chemical transformations.
For example, in the alkylation of aromatic compounds, KHF2 can be used to activate the alkylating agent, facilitating the transfer of the alkyl group to the aromatic ring. This catalytic role of KHF2 is particularly valuable in the production of fine chemicals, pharmaceuticals, and specialty materials, where precise control over reaction conditions is crucial.
Potassium Hydrogen Fluoride is a vital intermediate in the chemical industry, with applications spanning from fluorination reactions to the production of metal fluorides, hydrofluoric acid, and specialized chemicals. Its versatility and reactivity make it an essential component in numerous industrial processes, contributing to the development of advanced materials, pharmaceuticals, and more.




