The Application of Fluoroboric Acid in Copper Electroplating
Copper electroplating is an essential process in various industries, including electronics, automotive, and decorative arts. It involves depositing a thin layer of copper onto a substrate to enhance electrical conductivity, corrosion resistance, and aesthetic appeal. One critical component in this process is fluoroboric acid (HBF4). This article delves into the specifics of how fluoroboric acid is utilized in copper electroplating, highlighting its benefits and the underlying principles.
Introduction to Fluoroboric Acid
Fluoroboric acid, also known as tetrafluoroboric acid, is a strong, colorless acid composed of hydrogen, boron, and fluorine atoms. It is highly soluble in water, forming a stable and conductive solution ideal for use in electroplating processes. The unique chemical properties of fluoroboric acid make it particularly suitable for creating high-quality copper deposits.
Preparation of the Electrolyte Solution
The first step in copper electroplating with fluoroboric acid involves preparing the electrolyte solution. This solution typically contains:
● Fluoroboric Acid (HBF4): Provides the necessary acidity and ionic strength.
● Copper Fluoroborate (Cu(BF4)2): Supplies the copper ions required for plating.
● Additives: Such as brighteners, levelers, and wetting agents to enhance the quality of the copper deposit.
The preparation process involves dissolving the appropriate amounts of fluoroboric acid and copper fluoroborate in deionized water. The resulting solution is then filtered to remove any impurities that could interfere with the electroplating process.
Mechanism of Copper Electroplating with Fluoroboric Acid
The electroplating process involves two primary electrodes: the anode (usually made of pure copper) and the cathode (the substrate to be plated). When an electric current is applied, copper ions from the anode dissolve into the electrolyte solution and are subsequently deposited onto the cathode. The detailed steps are as follows:
1、Anodic Reaction:
● At the anode, copper metal dissolves into the solution as copper ions:
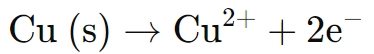
● The dissolved Cu2+ ions combine with fluoroborate ions (BF4-) from the fluoroboric acid to form stable copper fluoroborate complexes (Cu(BF4)2).
2、Cathodic Reaction:
● At the cathode, the copper ions in the electrolyte are reduced and deposited as a solid copper layer:
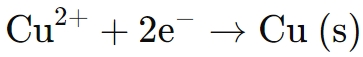
● The continuous supply of Cu2+ ions ensures a uniform and smooth deposition of copper onto the substrate.
3、pH and Conductivity Control:
● Fluoroboric acid helps maintain the pH of the electrolyte solution within the optimal range, preventing the formation of unwanted by-products such as copper hydroxide.
● The high ionic conductivity of the fluoroboric acid solution enhances the efficiency of the electroplating process, reducing internal resistance and allowing for better control over plating parameters.
Advantages of Using Fluoroboric Acid in Copper Electroplating
The use of fluoroboric acid in copper electroplating offers several distinct advantages that contribute to the overall quality and efficiency of the process:
1、High-Quality Copper Deposits:
Fluoroboric acid ensures the production of copper layers with excellent adhesion, smoothness, and uniformity. The deposits are free from common defects such as pitting, roughness, and nodules, which are often encountered with other electrolytes.
2、Enhanced Electroplating Efficiency:
The high conductivity and stable pH of the fluoroboric acid electrolyte result in increased plating efficiency. This leads to faster deposition rates and lower energy consumption, thereby reducing operational costs.
3、Versatility in Operating Conditions:
Fluoroboric acid-based electrolytes can operate effectively over a wide range of temperatures and current densities. This flexibility allows for customization of the electroplating process to meet specific application requirements.
4、Environmental and Safety Benefits:
Compared to other electroplating chemicals, fluoroboric acid is less hazardous and poses fewer environmental risks. It does not produce toxic by-products, making it a safer and more environmentally friendly choice.
5、Compatibility with Additives
Fluoroboric acid is compatible with various additives that improve the properties of the copper deposit, such as brighteners and levelers. These additives enhance the brightness, smoothness, and mechanical properties of the copper layer.
Industrial Applications of Copper Electroplating with Fluoroboric Acid
The use of fluoroboric acid in copper electroplating is prevalent in several industrial applications, each benefiting from the unique properties of the acid:

1、Electronics and Circuit Boards:
Copper electroplating is crucial in the manufacturing of printed circuit boards (PCBs). The high-quality copper deposits produced using fluoroboric acid ensure excellent electrical conductivity and reliable performance of electronic components.
2、Automotive Industry:
Copper-plated components in the automotive industry benefit from enhanced corrosion resistance and improved electrical connections. Fluoroboric acid ensures the durability and longevity of these components, even in harsh operating conditions.
3、Decorative Applications:
In the decorative arts, copper electroplating is used to create aesthetically pleasing finishes on various items, such as jewelry, watches, and household fixtures. The smooth and bright copper deposits produced with fluoroboric acid contribute to the visual appeal of these products.
4、Plumbing and Electrical Systems:
Copper-plated pipes and connectors in plumbing and electrical systems benefit from the enhanced corrosion resistance provided by fluoroboric acid-based electroplating. This extends the service life of these components and ensures reliable performance.
Fluoroboric acid plays a crucial role in the copper electroplating process, offering significant benefits in terms of quality, efficiency, and versatility. By stabilizing copper ions, enhancing electrolyte conductivity, and maintaining optimal pH levels, fluoroboric acid ensures the deposition of high-quality copper layers with excellent properties. Its application spans numerous industries, contributing to the advancement of technologies and the production of superior products.




