Introduction of Ammonium fluoride
Molecular Formula: FH4N or NH4F
Synonyms: 12125-01-8,Ammonium fluoride ((NH4)F),Fluoruro amonico,Ammonium fluorure,Fluorure d'ammonium
Molecular Weight :37.037
What is ammonium fluoride considered?
Ammonium fluoride is a white crystalline solid. It is soluble in water. It is noncombustible. It is corrosive to aluminum. It is used in chemical analysis, in brewing, and as a preservative for wood.
It is also used in printing and dyeing textiles, glass etching, mothproofing and wood preserving. * Ammonium Fluoride is on the Hazardous Substance List because it is regulated by OSHA and cited by ACGIH, DOT, NIOSH and EPA.
Ammonium fluoride is a fluoride salt having ammonium (NH4+) as the counterion. It is an ammonium salt and a fluoride salt.
Is ammonium fluoride an acid?
Ammonium fluoride will react with water (including perspiration) and may form hydrofluoric acid.
Chemical Structure Depiction: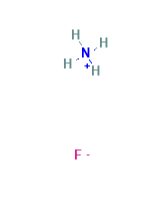
CAS Number:12125-01-8
DOT Hazard Label:Poison
Air & Water Reactions
Dissolves in water and forms dilute solution of hydrofluoric acid. May corrode glass, cement, and most metals (USCG, 1999). Water soluble.
Fire Hazard
Special Hazards of Combustion Products: Toxic ammonia and hydrogen fluoride gases are formed in fires.
Behavior in Fire: May sublime when hot and condense on cool surfaces (USCG, 1999)
Health Hazard
Inhalation of dust may cause irritation of respiratory system. Ingestion is harmful; readily soluble fluorides may be fatal if relatively small quantities are swallowed. Contact with eyes causes local irritation of the mucous membrane. Contact with skin may cause burns. High concs. of fluorine in the urine have been reported following skin contact. (USCG, 1999)
Reactivity Profile
Ammonium fluoride reacts with water to form hydrofluoric acid, a source of fluoride ions. Unlike other halide ions, the fluoride ion is quite reactive, acting as a weak base and participating in some unique reactions. In particular, fluorides react strongly with compounds containing calcium, magnesium, or silicon ions, which means that solutions containing soluble fluorides are corrosive to both living tissue and glass. Hydrofluoric acid can cause severe chemical burns and is one of the few materials that can etch glass. It is also a toxic gas in its anhydrous form.
Potentially Incompatible Absorbents
Use caution: Liquids with this reactive group classification have been known to react with the absorbents listed below. More info about absorbents, including situations to watch out for...
• Mineral-Based & Clay-Based Absorbents
• Sand
• Dirt/Earth
We supply FH4N focus on quality, service and expertise:
• Quality: Producing purity synthetic FH4N, based on pure raw materials only, with the lowest heavy metal content. We can also manufacture products of different concentrations to meet customer needs.
• Service: Offer stable quality of the product to clients, keep doing best for after-sales service.A close cooperation with our clients enables us tobetter understand the customer's usage of our products, and make modifications according to the customer's feedback .
•Expertise: Custom-made packaging and logistics、different concentrations of chemical products can be offered to meet customer’s requirements.
FOSHAN NANHAI SHUANGFU CHEMICAL CO., LTD is one of the manufacture of medium and high quality Potassium tetrafluoroborate, supply long term production and delivery of this product all over the world.
Attn:Nicole Huang (Sales Manager)
Add: Qishan Industry Park, Yanfeng Road, Shishan, Nanhai District,Foshan City,China.
Phone: 86 757 81108788
Mobile: 86 13928083637
E-mail: sales@df-chemicals.com
- Fluoride Salt
- Ammonium Fluoride
- Sodium Fluoride
- Potassium Fluoride
- Sodium Hydrogenfluoride
- Potassium Bifluoride
- Magnesium Fluoride
- Aluminium Fluoride
- Barium Fluoride
- Lithium Fluoride
- Strontium Fluoride
- Nickel Fluoride
- Zinc Fluoride




