Ammonium Fluoroborate as an Electrolyte in Various Industries
Ammonium fluoroborate, also known as NH4BF4, has become a widely utilized chemical compound in various industries due to its exceptional properties as an electrolyte. It is an ideal option for numerous electrochemical processes, including metal surface treatments and battery production. This article will explore the process of ammonium fluoroborate becoming an electrolyte and its broad usage across different sectors.
Formation of Ammonium Fluoroborate as an Electrolyte:
Ammonium fluoroborate is typically synthesized through the reaction between ammonium hydroxide (NH4OH) and hydrofluoric acid (HF), followed by the addition of boric acid (H3BO3). The chemical equation for this reaction is:
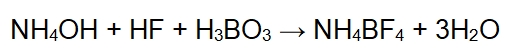
In this reaction, the ammonium ion (NH4+) from ammonium hydroxide combines with the fluoride ion (F-) from hydrofluoric acid, forming the ammonium fluoroborate salt. This salt dissociates in solution, producing ammonium (NH4+) and fluoride (F-) ions, which make it an electrolyte.
Applications of Ammonium Fluoroborate Electrolyte:
1、Electroplating:
Ammonium fluoroborate serves as a key component in electroplating processes, where a metal coating is deposited onto a conductive surface. The electrolyte facilitates the transfer of metal ions from the plating solution onto the substrate, creating a uniform and adherent metal coating. This technique is widely employed in industries such as automotive, electronics, and jewelry manufacturing.

2、Surface Treatment:
In industries requiring surface treatment for enhanced corrosion resistance or improved aesthetics, ammonium fluoroborate-based electrolytes are utilized in processes like anodizing and chemical conversion coatings. These treatments modify the surface properties of metal substrates, providing them with desirable characteristics for various applications.
3、Battery Manufacturing:
Ammonium fluoroborate finds application as an electrolyte additive in certain types of batteries, including lithium-ion batteries. Its role involves enhancing battery performance and stability by optimizing electrolyte conductivity and suppressing undesirable side reactions. This application contributes to advancements in portable electronics, electric vehicles, and renewable energy storage systems.

4、Chemical Synthesis:
Beyond electrochemical applications, ammonium fluoroborate is also employed in organic synthesis as a fluorinating agent. It participates in reactions involving the introduction of fluorine atoms into organic molecules, facilitating the synthesis of fluorinated compounds used in pharmaceuticals, agrochemicals, and materials science.
In summary, ammonium fluoroborate plays a vital role as an electrolyte in diverse industrial applications, particularly in electroplating and metal finishing processes. Its ability to facilitate uniform coating, enhance adhesion, and ensure high-quality finishes makes it a preferred choice in many sectors. Additionally, its relatively low toxicity adds to its appeal as a safer alternative in industrial settings.
- Fluoride Salt
- Ammonium Fluoride
- Sodium Fluoride
- Potassium Fluoride
- Sodium Hydrogenfluoride
- Potassium Bifluoride
- Magnesium Fluoride
- Aluminium Fluoride
- Barium Fluoride
- Lithium Fluoride
- Strontium Fluoride
- Nickel Fluoride
- Zinc Fluoride




