Borofluoric Acid as a Catalyst: Applications and Mechanisms in Borofluoride Salt Formation
Borofluoric acid (HBF4) is a highly reactive inorganic acid that plays an important role in modern chemistry and industry. When used as a catalyst, HBF4 facilitates a wide variety of reactions, particularly those that involve the formation of borofluoride salts, a class of compounds that are useful in various industrial applications. In this article, we will explore the specific applications of borofluoric acid as a catalyst, its role in facilitating chemical reactions, and the underlying mechanisms that make it such a versatile and effective catalyst.
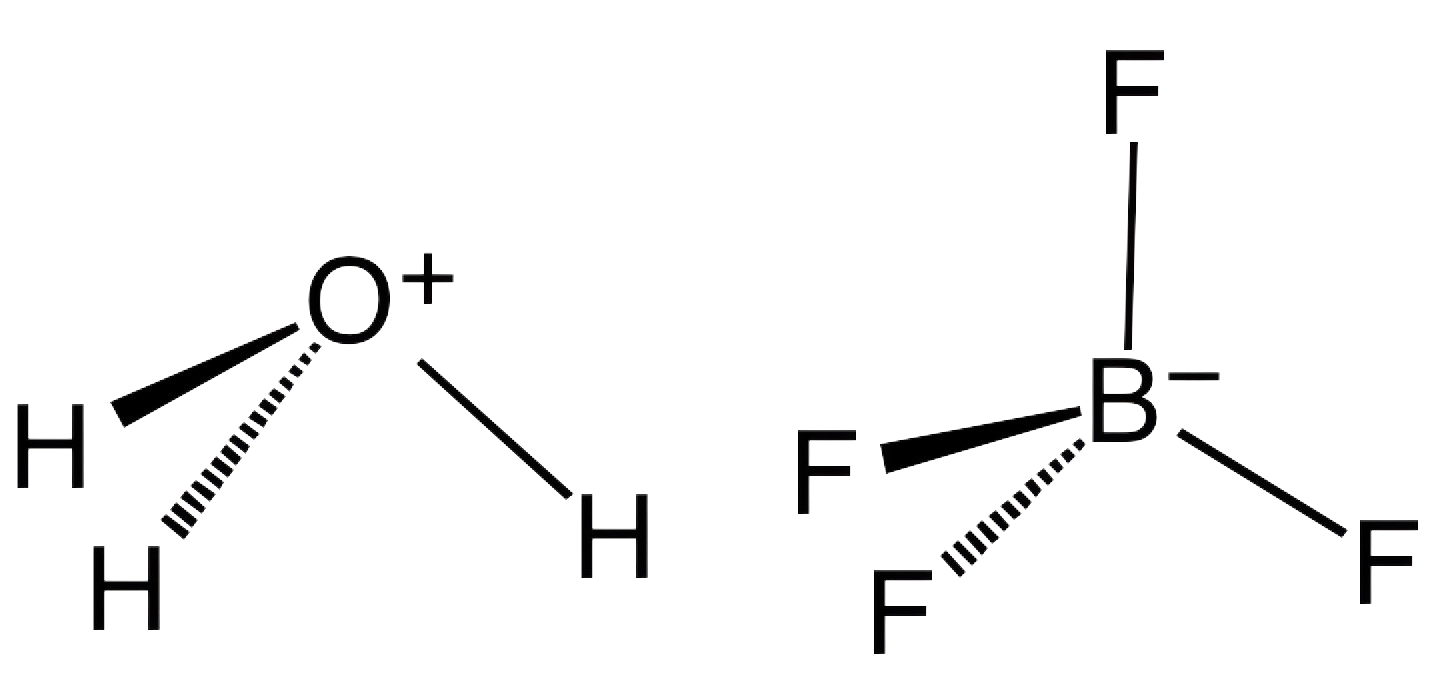
1. What is Borofluoric Acid and How Does It Work as a Catalyst?
Borofluoric acid, with the chemical formula HBF4, is a compound consisting of hydrogen, boron, and fluorine. It is a strong acid that dissociates in solution to release a proton (H+) and the tetrafluoroborate anion (BF4-). The unique combination of boron and fluorine in its structure gives it distinct catalytic properties, particularly in reactions where fluorine-based species or boron chemistry is involved.
As a catalyst, borofluoric acid functions by promoting certain chemical reactions without being consumed in the process. It can activate molecules by donating protons (acting as a Brønsted acid) or by accepting electron pairs (acting as a Lewis acid). These properties make HBF4 particularly useful in various reactions, including the formation of borofluoride salts, which have broad industrial applications.
2、Applications of Borofluoric Acid as a Catalyst
2.1 Formation of Borofluoride Salts
One of the most common uses of borofluoric acid is in the synthesis of borofluoride salts, such as sodium tetrafluoroborate (NaBF4), potassium tetrafluoroborate (KBF4), and ammonium tetrafluoroborate (NH4BF4). These salts are crucial in several chemical processes and are formed by neutralizing borofluoric acid with a suitable base. The general reaction is as follows:
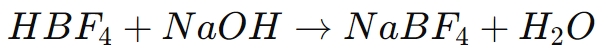
In this process, borofluoric acid donates a proton to the base, forming water and leaving behind the tetrafluoroborate anion (BF4-), which then associates with the metal ion (e.g., Na+) to form a borofluoride salt.
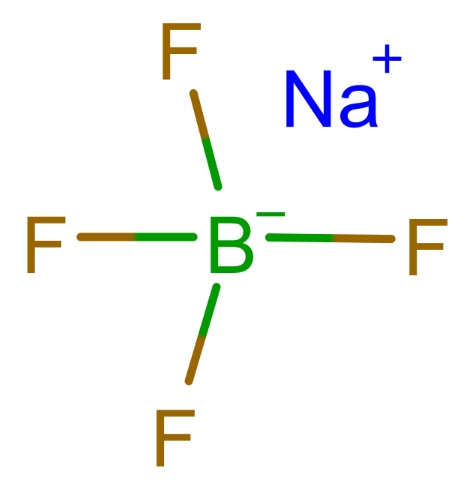
These borofluoride salts are used in various fields, including:
● Electrochemistry: Used as electrolytes in lithium-ion batteries and electroplating processes.
● Polymer Chemistry: Act as initiators in polymerization reactions.
● Fluorination: Used to introduce fluorine atoms into organic compounds, enhancing their stability and reactivity.
2.2 Organic Synthesis and Catalysis
Borofluoric acid also plays a critical role in organic synthesis, particularly in the activation of electrophilic reagents. When used as a catalyst, HBF4 can accelerate reactions such as Friedel-Crafts alkylation, acylation, and other electrophilic substitution reactions. The mechanism typically involves the Lewis acid properties of HBF4, where it coordinates with electron-rich species, enhancing their reactivity and promoting the formation of new bonds.
For example, in the Friedel-Crafts alkylation, HBF₄ activates alkyl halides, facilitating the addition of alkyl groups to aromatic rings. The general reaction for such a process is:
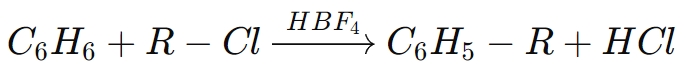
In this reaction, borofluoric acid acts as a catalyst to facilitate the alkylation of benzene with an alkyl chloride, producing an alkylated aromatic compound.
2.3 Polymerization and Oligomerization
HBF4 is also an effective catalyst for polymerization reactions. In particular, it is used to initiate the polymerization of styrene and other monomers, leading to the formation of valuable polymer materials like polystyrene. Borofluoric acid can activate the double bond in the monomer, allowing for the growth of polymer chains and the formation of the polymer.
For example, in the polymerization of styrene (C8H8):
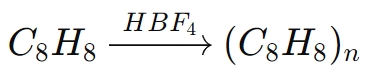
In this reaction, HBF4 initiates the polymerization by donating a proton to the styrene molecule, generating a reactive carbocation, which then propagates the polymer chain.
Moreover, borofluoric acid can catalyze oligomerization, the process where small molecules (oligomers) form longer chains without full polymerization. This is important in the production of specialized materials used in coatings and adhesives.
2.4 Electrochemical and Battery Applications
In the field of electrochemistry, borofluoric acid is widely used to create borofluoride salts that serve as electrolytes in lithium-ion and other types of batteries. The use of borofluoride salts in batteries improves their ionic conductivity and stability, making them highly suitable for use in energy storage systems.
For example, lithium tetrafluoroborate (LiBF4) is commonly used as an electrolyte in lithium-ion batteries because of its high ionic conductivity and stability at high voltages.
Borofluoric acid's role in electrochemical processes extends to metal electroplating, where borofluoride salts act as electrolyte solutions that facilitate the deposition of metals such as copper, gold, and tin. The use of borofluoric acid in this context helps to create more uniform coatings on metals, making it an essential component in industries like electronics and metal finishing.
2.5 Fluorination of Organic Compounds
Another significant application of borofluoric acid as a catalyst is in fluorination reactions. The ability to incorporate fluorine atoms into organic molecules is valuable in the production of pharmaceuticals, agrochemicals, and specialty materials. Borofluoric acid can activate fluorine sources, such as fluorine gas or fluorine-containing compounds, enabling them to react with organic substrates.
For example, in the fluorination of an organic compound (R-X):
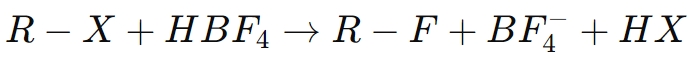
Here, borofluoric acid helps facilitate the substitution of a halogen atom with fluorine, improving the properties of the resulting compound, such as its chemical stability and resistance to degradation.
3、Mechanism of Catalysis by Borofluoric Acid
The catalytic activity of borofluoric acid can be explained by its ability to function as both a Brønsted acid and a Lewis acid:
● Brønsted Acid: As a proton donor, borofluoric acid can protonate substrates, increasing their electrophilicity and making them more reactive in subsequent reactions. This is particularly useful in reactions like nucleophilic substitution, where an electron-rich nucleophile attacks an electrophilic substrate.
● Lewis Acid: Borofluoric acid acts as a Lewis acid by accepting electron pairs from nucleophilic species, thereby activating electrophiles and facilitating various substitution and addition reactions. This is especially evident in reactions like Friedel-Crafts alkylation, where HBF₄ coordinates with alkyl halides, making them more reactive with aromatic compounds.
Through these mechanisms, borofluoric acid can lower the activation energy of reactions, increase reaction rates, and improve the yield of desired products.
4、Advantages and Challenges of Using Borofluoric Acid
4.1 Advantages
● Efficiency: Borofluoric acid accelerates reactions by lowering activation energy, resulting in faster reaction times and higher yields.
● Selectivity: Its strong acidic properties ensure that only specific substrates are activated, providing high selectivity in complex reactions.
● Versatility: It can be used in a wide range of reactions, including organic synthesis, polymerization, and electrochemical processes.
4.2 Challenges
● Corrosiveness: Due to its strong acidic nature, borofluoric acid is highly corrosive and requires specialized handling and equipment.
● Environmental Impact: As a fluorine-containing compound, borofluoric acid and its derivatives must be carefully disposed of to avoid environmental contamination.
● Cost: The production and purification of borofluoric acid can be costly, limiting its use in large-scale industrial processes.
Borofluoric acid is an incredibly versatile catalyst that plays a critical role in the synthesis of borofluoride salts, organic synthesis, polymerization, and electrochemical applications. Its ability to act as both a Brønsted and Lewis acid makes it an indispensable tool in many chemical processes. However, its use requires careful handling due to its corrosive nature and environmental impact. With ongoing research and technological advancements, borofluoric acid is likely to find even more applications in various industries, driving innovation and efficiency across a wide range of sectors.




