Lithium Fluoride CAS 7789-24-4

- FSSF
- China
- in 5 days
- 3000tons/month
Double Fluoride Chemical's Lithium Fluoride is mainly used in:
1. It is the basic component of fluorine electrolytic cell electrolyte. In high-temperature storage batteries in a molten state as the electrolyte component.
2. Used as a flux in the production of ceramics.
3. Used in enamels, glasses and glazes.
4. Used in brazing and welding fluxes and molten salt chemistry in metallurgy.
5. Optical glass and battery.
6. Additive in aluminum-electrolysis melts.
7. Raw material for optical lenses and prisms.
LITHIUM FLUORIDE CAS NO.: 7789-24-4
lithium fluoride
Molecular Formula: LiF
Mol. Wt: 25.94
Cas No.: 7789-24-4
UN No.: 3288
EINECS: 208-407-7
Synonyms: Fluorolithium, Lithium monofluoride, Lithium fluoride (LiF)
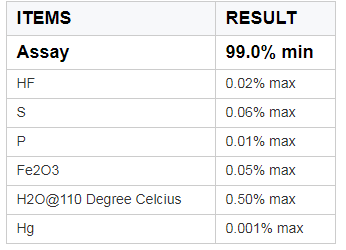
Product image:

Physical state: White to off white random crystals
Melting point: 845 °C (lit.)
Boiling point: 1681 °C
Stability: Stable, but hygroscopic. Hydrolyzes in the presence of water to form hydrofluoric acid, which attacks glass - do not store in glass bottles. Incompatible with aqueous solutions, strong acids, strong oxidizing agents.
Hazardous characteristics: It decomposes in acid and emits corrosive hydrogen fluoride gas. Decomposes highly toxic fumes when exposed to high temperatures.








